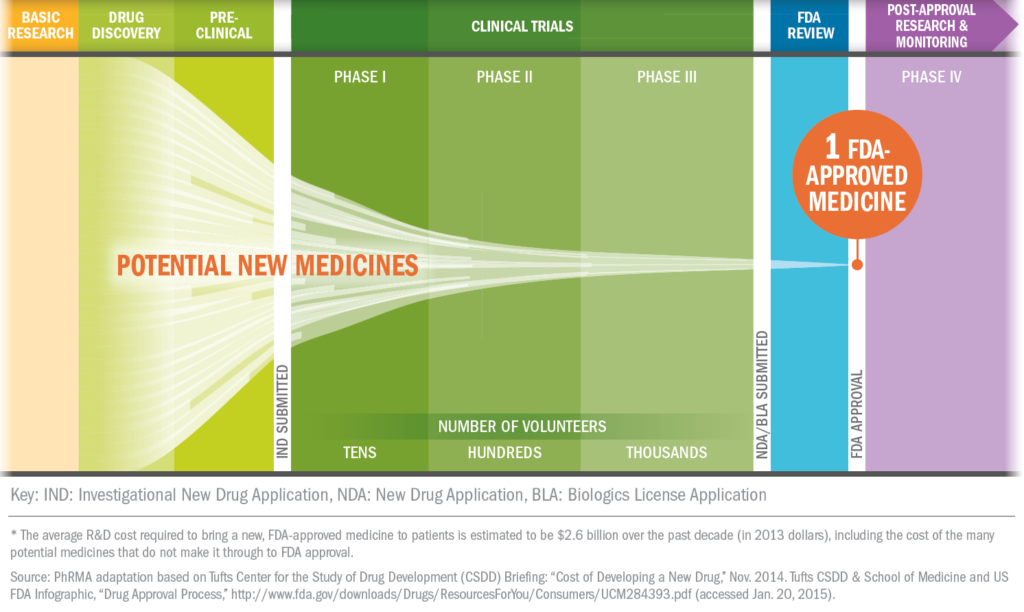
About Clinical Trials
What's it like to participate in a clinical trial?
Some clinical trials are conducted to compare existing treatments and some are done to explore whether a drug is appropriate for a different patient population, such as children or the elderly. Still others are conducted to find ways to make existing approved drugs more effective and easier to use with fewer side effects.
For patients, clinical trials offer the potential for another therapeutic option. Clinical trials may provide a new avenue of care for some chronic disease sufferers who are still searching for the medicines that are best for them.
Diversity and Clinical Trials
As a nation, we are in a new era of medicine where breakthrough science is transforming patient care, but these innovations are meaningless if they don’t reach all patients. It is critical that patients from traditionally underserved communities have access to innovative medicines. Achieving health equity is essential in creating a health care system that truly works.
Systemic racism that exacerbates health inequities has contributed to long-standing disparities in prevalence and severity of disease across racial and ethnic groups. These disparities can reflect in how often a disease occurs in a certain patient population, how serious the disease manifests itself in patients or how often a disease results in death.
Health disparities have many causes, including limited access to quality health care, health screenings, living and working conditions, experiences with the health care system/patient confidence, racism, bias in the treatment setting, underrepresentation of minority health care providers, and other social determinants of health, clinical trial participation, language barriers, and economics and insurance coverage.
The research-based biopharmaceutical industry recognizes the importance of including diverse patients in clinical trials for new medicines so that the clinical trial population reflects the intended treatment population. Addressing the systemic issues that deter Black and Hispanic communities from participating in clinical trials is critical to enhancing clinical trial diversity so that those who want to participate, can.
Underrepresentation of racial and ethnic groups in clinical trials for new medicines has a long history. In an effort to address this long-standing mistrust and other issues, PhRMA and its member companies recently issued the first-ever industry-wide principles on clinical trials diversity, adding a new chapter to the already existing Principles on Conduct of Clinical Trials & Communication of Clinical Trial Results. The new clinical trial diversity principles address:
- Building Trust and Acknowledging Past Wrongs
- Reducing Barriers to Clinical Trial Access
- Using Real-World Data to Enhance Information on Diverse Populations Beyond Product Approval
- Enhancing Information About Diversity and Inclusion in Clinical Trial Participation
Patient Resources
Finding Clinical Trials in Wisconsin
Patients can learn about clinical trials in several ways. Health care providers are aware of clinical trials being conducted at hospitals, universities, and other leading health care facilities, and these institutions can be valuable sources of information for patients looking to participate.
More information about clinical trials in Wisconsin and how to volunteer for one can be found at www.centerwatch.com.
Patients can also use hospital and university websites to find trials being conducted in their area.
Wisconsin's Patient Advocacy Groups
Patient advocacy groups in Wisconsin provide an exceptional resource for patients to connect and learn more about their condition and what treatment options are available in the state. These groups also provide an important voice on behalf of patients to protect their access to medicine and treatment.
The following are just a few major groups that work on behalf of patients in Wisconsin and may provide more information to patients with further questions.
Alzheimer’s Association
Chippewa Valley Office
404 1/2 N. Bridge Street
Chippewa Falls, WI 54729
(715) 720-7611
Alzheimer’s Association
Green Bay Office
(920) 469-2110
Alzheimer’s Association
La Crosse Office
3817 Mormon Coulee Road, Suite B
La Crosse, WI 44601
(800) 272-3900
Alzheimer’s Association
Madison Office
2820 Walton Commons, Suite 132
Madison, WI 53718
(608) 203-8500
Alzheimer’s Association
Milwaukee Office
620 S. 76th Street, Suite 160
Milwaukee, WI 53214
(414) 479-8800
Alzheimer’s Association
Rhinelander Office
8A W. Davenport Street, Suite 224
Rhinelander, WI 54501
(715) 362-7779
Alzheimer’s Association
Wausau Office
(715) 803-6779
Alzheimer’s and Dementia Alliance of Wisconsin
3330 University Avenue, Suite 300
Madison, WI 53705
(608) 232-3400 or (888) 308-6251
American Cancer Society
Wisconsin Office
P.O Box 902
Pewaukee, WI 53072
(800) 227-2345
American Diabetes Association
Milwaukee Office
P.O. Box 7023
Merrifield, VA 22116-7023
(414) 778-5500
adawi@diabetes.org
American Heart Association
Milwaukee Office
1555 N. RiverCenter Drive, Suite 211
Milwaukee, WI 53212
(414) 271-9999
American Heart Association
Madison Office
2850 Dairy Drive, Suite 300
Madison, WI 53718
(608) 709-4930
American Liver Foundation
Wisconsin Resource Center
1845 N. Farwell Avenue, Suite 312
Milwaukee, WI 53202
(414) 763-3435
American Lung Association
Wisconsin Chapter
13100 W. Lisbon Road, Suite 70
Brookfield, WI 53005
(262) 703-4200
Arthritis Foundation
Wisconsin Chapter
10427 W. Lincoln Avenue, Suite 1300
West Allis, WI 53227
(414) 533-0453
Coalition of WI Aging & Health Groups
30 West Mifflin, Suite 406
Madison, WI 53703
(608) 224-0606
Epilepsy Foundation of Wisconsin
41 Park Ridge Drive, Suite C
Stevens Point, WI 54481
(608) 665-1848
NAMI Wisconsin
National Alliance on Mental Illness
4233 W. Beltline Hwy.
Madison, WI 53711
(608) 268-6000
Additional Resources
![]()
Medicine Assistance Tool (MAT)
The Medicine Assistance Tool, a PhRMA-sponsored web platform designed to help patients, caregivers and health care providers learn more about the resources available through the various biopharmaceutical industry programs offered to those who need financial support due to their lack of insurance or inadequate prescription medicine coverage. MAT is not its own patient assistance program, but rather, a search engine for many of the support programs and resources that the biopharmaceutical industry has been offering for decades. Patients should go to www.mat.org for more information. The on-line process takes about 15 minutes, and you’ll find out instantly if you’re likely to be eligible for help. Learn more at www.mat.org

Healthcare Ready
Healthcare Ready is a tool activated to help keep emergency responders informed on the status of the biopharmaceutical supply chain in the event of a natural disaster or emergency. Healthcare Ready’s Rx Open tool has been deployed in 11 states and the District of Columbia and helped victims and evacuees who needed to fill or re-fill their prescriptions find open pharmacies. Healthcare Ready also helped emergency responders with critical information on the challenges facing supply chain partners relating to electricity, fuel and transportation issues. See more at www.healthcareready.org